A host of regional bread specialties has long marked the German-speaking realm as a sort of bread paradise, one truly unique in the world. Let us therefore pursue creation of a loaf of German bread, from rye and wheat field all the way to the table, taking time as we go to note perhaps with some surprise the numerous chemical processes that unfold before us.
4. Preparing the Dough for a Wheat-based Bread
Before actually preparing a dough, the flour is first sifted, and thereby loosened up and aerated. Oxygen introduced in this way contributes to rapid aerobic growth and proliferation of the yeast to be employed.
The various conditions of mixing and kneading of the ingredients, up to the onset of baking, (i.e., temperature, moisture, recipe, intensity and duration of kneading, and rest periods) taken together describe a specific “dough process”. This begins with the addition of water to wheat flour, salt, and yeast (Fig. 5). An initially sticky, unshapeable mass (Fig. 6) is transformed through constant kneading into a soft, smooth, and—after a rest period—readily shapeable dough (Fig. 7).

Figure 5. Addition of water to wheat flour, salt, and yeast. © Schweizerische Brotinformation (SBI), Bern, Switzerland.

Figure 6. A sticky, non-moldable dough mixture. © Schweizerische Brotinformation (SBI), Bern, Switzerland.

Figure 7. Kneading produces a soft, smooth dough. © Schweizerische Brotinformation (SBI), Bern, Switzerland.
What Lies Behind this Change?
The astonishing dough transformation cannot be due simply to the chemical constitution of the flour, since all cereal flours are in this respect similar, and it is seen only with wheat flour. No other cereal is the source of such an elastic dough. In order to understand the actual cause we must look closely at the molecular consequences of dough kneading.
4.1 Adhesive
After water has been added to flour, water molecules are attracted to the polar side-chains of proteins, severing hydrogen bonds between proteins and hydroxyl groups of the starches. Protein chains set free in this way experience mutual attraction by way of hydrophobic interactions, producing an adhesive: gluten. The term “adhesive” here is apt, since after washing the starch out of a dough, the former can be isolated as a rubber-like, sticky mass.
This gluten is 90 % protein, 8 % lipid, and 2 % carbohydrate. The protein fraction is composed of roughly equal parts of a mixture of glutenin and gliadin. Gliadins are monomers, and are stabilized in their compact form by intramolecular disulfide bridges. In contrast to this, glutenin contains many subunits coupled near their ends through intermolecular disulfide bridges, leading to long, extended chains.
In the case of all glutenin subunits so far examined, one finds at position 10 counting from the N-terminus a cysteine unit [7]. Kneading of the dough causes disulfide bridges to be repeatedly severed and then oxidatively re-established until a three-dimensional glutenin network forms such that embedded within it are the globular gliadins.
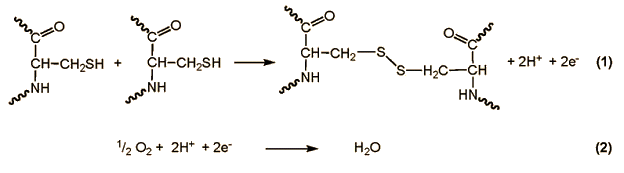
Through pulling on the dough in this way, the structural elements of the long glutenin chains, which to some extent display pleated-sheet character, are drawn apart, but after relaxation of the external force these tend to regain some measure of their original form; that is, glutenin confers upon dough a certain degree of elasticity.
The globular gliadins lie between the glutenin chains, where they function a bit like ball-bearings, and facilitate displacement of the chains relative to one another. As a result, they introduce plasticity into a dough.
4.2 Lipids
The unique characteristics of wheat-flour dough are, however, not due exclusively to the presence of gluten, with its adhesive properties, but to other substance classes as well. Thus, lipids stabilize the formation of pores, as a dough formed with flour from which all fats have been eliminated shows significantly less increase in volume in the course of baking. It is, above all, polar lipids that play a role here. These contain in addition to one or two hydrophobic fatty-acid chains a polar, hydrophilic molecular segment, which introduces appreciable emulsifying and surfactant character [8]. Moreover, formation during the kneading process of small fat droplets (liposomes, with a diameter of ca. 100 mm) introduces “lubricant” properties, thereby increasing the ability of a dough to “stretch”.
4.3 “Rest Periods”, and “Working” a Dough
Freshly kneaded dough tends inevitably to contract, preventing it from readily being formed. In the course of a “rest period” of 15–60 minutes the glutens in the dough “relax”, while the yeast continues to ferment, and rising proceeds. The latter facilitates formation of aroma-bearing substances. Careful compression of the dough subsequent to “resting” (“working”) causes the destruction of larger gas bubbles present, which leads in turn to a certain degree of uniformity among those bubbles that remain.
5. Preparation of Dough for a Rye Bread
One does not knead a rye dough; the ingredients are simply mixed, because the dough produced from a rye flour is not elastic. The content of gluten proteins is simply too low to support elasticity. The fact that rye dough rises, and indeed its very cohesion, is due to the presence of pentosans (Fig. 8), a class of complex polysaccharides with a certain amount of protein content. These constitute only 2–3 wt.-% in the case of wheat flour, but 6–8 wt.-% with rye.
In the case of wheat, pentosans are found largely in the hull or bran, and are mostly separated out during the milling of flour, whereas with rye the pentosans are distributed throughout the kernels, and thus remain after milling. Flours from other types of grain, such as rice, maize, oats, millet, or barley, are not well suited to bread production, and must therefore be mixed into wheat or rye doughs. Neither the “adhesive” proteins, as in wheat flour, nor the pentosans of rye are present here to a sufficient extent, so that the resulting dough is too soft, and lacks the potential for adequate gas retention, prerequisite for leavening: causing a dough to become “lighter” during baking (see below).
Pentosans bind a large amount of water, which is not released either during or after baking. This explains why the crumb of rye bread has a moister texture than that of wheat bread, remaining fresh longer without drying out rapidly.
A pentosan has an extremely complex structure, one which can be rendered only in representative form. The backbone consists of polysaccharide xylose chains (Fig. 8, blue, 5), known as “xylans”. On these xylan chains are short arabinose side chains (red, 6). Xylan chains bearing such arabinose branches are referred to as “arabinoxy chains”; the corresponding water solubility increases with increasing arabinose content. Multiple arabinoxy side chains are often interconnected by way of diferulic acid (black, 8), via ester linkages, and additionally through ferulic acid (7, magenta) via SH-side chains of cysteine units from proteins (green).
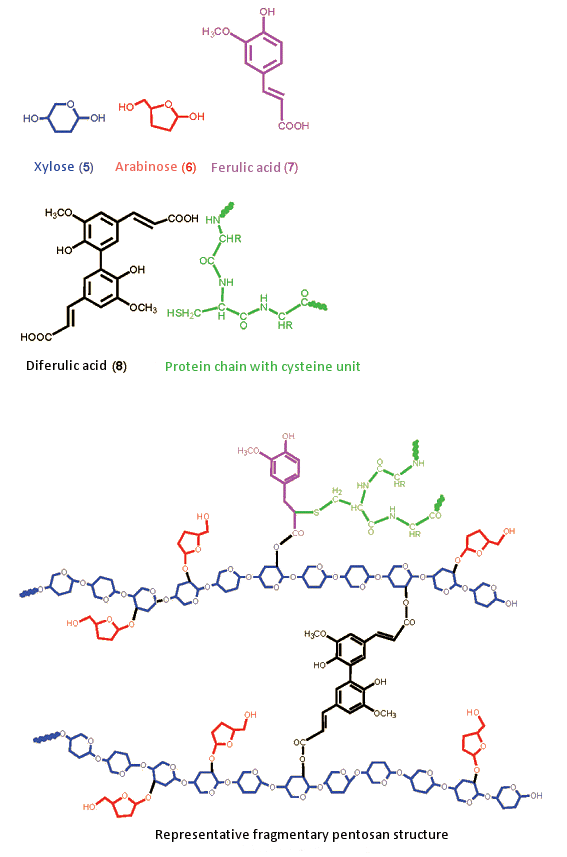
Figure 8. Representative model of a pentosan.
Both types of phenolic linkage can be oxidized by oxygen in the air, and they interlink the xylan chains such that overall viscosity increases. Moreover, proteins become attached by way of the side chains. The entire pentosan-protein complex has been described as resembling a glue, or a rubbery fabric. Insoluble pentosans primarily determine the properties of a rye dough.
6. Leavening
Attempts to bake a “bread” in the absence of a leavening agent lead only to a thin, dense, flat product like a tortilla or a crepe. Discovery of the first leavening agent represented a major breakthrough for our ancestors. It was probably a chance discovery resulting from forgetting to bake a fresh batch of dough, and not putting it in the oven until the next day, only to find that the resulting bread was much lighter and “airier” than usual, and tasted better as well. In the course of millennia, two leavening agents have established themselves as especially reliable: yeast and sourdough.
Further possibilities for “fluffing up” a bread dough include introduction of a rising agent such as baking powder: sodium hydrogen carbonate, which releases CO2 in the presence of an acid, like sodium dihydrogen phosphate. Some alternative leavening agents are especially suited to heavier doughs, like those for cookies, including potassium carbonate or the thermally unstable mixture of ammonium hydrogen carbonate and the corresponding carbamate. Flat breads without leavening are common in many cultures, as for example Mexican tortillas and the roti breads of India and Southeast Asia (chapati, phulka, etc.).
6.1 Yeast Fermentation
Yeast fermentation may begin through a dough simply being allowed to stand for a while, or after addition of a dough residue from the previous day. The invention of compressed baker’s yeast in 1847 by the Vienna brewer Adolf Ignaz simplified the task of bread bakers, since it made possible the introduction of a precisely defined quantity of high-quality yeast into a batch of dough.
Today, depending on the recipe and local custom or preference, the entire amount of yeast might either be added at the outset (direct method; i.e., all the ingredients are mixed together at once), or the dough might be induced to rise at first with just a small starter amount of yeast, and in any of a variety of ways (e.g., biga, poolish, etc.), and processed further only after this starter yeast has been provided with a sufficiently long growth phase (indirect methods). For the initial aerobic yeast-cell growth phase it is advantageous to ensure that, prior to mixing, the flour has been sifted, a precaution that achieves aeration.
6.2 Sourdough
Rye doughs are loosened up with the aid of sourdough. Whereas in the case of wheat where there is likely to be too little amylase present for adequate degradation of starches, in rye flour there is inevitably too much. This amylase excess would be the cause of too much starch degradation during baking, leading to a dough that is much too soft, and from which it is impossible to bake a tasty bread.
Addition of sourdough lowers the pH of the dough to a value below 5, which significantly reduces amylase activity. Only then does rye flour lend itself to baking. In the absence of acidification, the crumb lacks elasticity, and chewing causes the crumb to agglomerate. Such bread tastes bland, and smells a bit musty and like cardboard; also, each loaf ends up consisting largely of one big hole (Fig. 9, D).
.jpg)
Figure 9. Bread mistakes, and their causes. © UNIFERM GmbH & Co. KG, Werne, Germany.
A) Enzyme-deficient wheat flour: too little starch is decomposed in the course of baking, so the dough is too firm, and the crumb leaves an impression when chewed of being too firm and dry. Moreover, it tastes bland and doesn’t seem fresh.
B) Wheat flour rich in enzymes: Too much decomposition of starch causes the dough to be too soft, and the bread shows too little cohesion. Due to insufficient capacity for gas retention by the soft dough, the pores are very irregular, and it is difficult to apply a spread to the slices.
C) Enzyme-deficient rye flour: With rye flour, if a sufficient supply of enzymes is lacking, or the dough is too acidic, very little starch is decomposed. The dough becomes too firm, and the resulting bread seems soggy and offers little evidence of freshness.
D) Rye flour rich in enzymes: If too much starch is decomposed due either to an excess of enzymes in the rye flour or too little acidification, too much carbon dioxide is released in a dough that is too soft. Cavities form in the bread, and the bread itself lacks elasticity and tends toward formation of soggy masses.
7. How Does One Create a Sourdough?
A dough prepared from rye flour and water is allowed to stand for several hours until it spontaneously begins to ferment. Through gradual addition of flour and water, this fermentation can be encouraged to proceed, such that the desired microorganisms evolve further. The latter include various species of lactic acid bacteria (Lactobacillus sanfranciscensis, L. brevis, L. plantarum, L. delbrückii), which derive their energy from anaerobic degradation of carbohydrates to carbon dioxide and either lactic acid alone (homofermentative) or a combination of lactic and acetic acids (heterofermentative).
This fermentation and gas formation, which in the beginning is only weakly acidic, gradually becomes more vigorous, with sourdough yeasts and lactic acid bacteria gaining the upper hand, and undesirable microorganisms being gradually displaced. Sourdough is a microbiological biotope of great versatility, constituting a science in itself.
Due to the risk that undesirable microorganisms in a sourdough culture might over time multiply too rapidly, as for example Escherichia coli bacteria, bakers renew their sourdough at regular intervals using a fresh starter obtained from a professional distributer.
Sourdough serves not only to lower the pH during fermentation: it also confers on bread an acidic note, and a characteristic aroma. It is therefore not surprising that sourdough is a feature of many recipes where it is not actually essential from the standpoint of baking technology, but is introduced simply for flavoring purposes.
8. Chemistry of the Baking Process
Although the only new factor introduced during baking is an increase in temperature (Tab. 3), the equivalent of a Concerto grosso nonetheless plays itself out here, culminating in a brilliant finale.
Table 3. Conditions for baking various types of bread.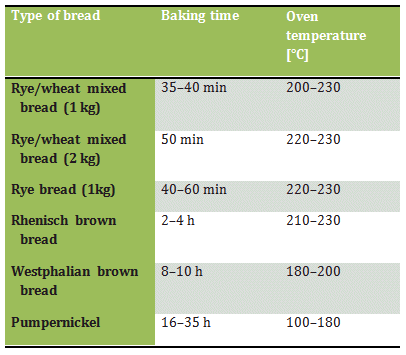
The beginning is rather unspectacular: loaves are simply introduced into the oven, which soon fills with steam, and water begins to condense on the cold surface of the dough. The heat of condensation thereby released ensures rapid heating of the clumps of dough, and steam prevents the outermost layers of dough from drying out prematurely. This ensures that they remain elastic, and will not crack during the expansion process. Continued increase in the temperature leads to denaturing of the bread’s proteins, along with gelatinization of starches and the formation of gas bubbles from steam trapped in the gradually solidifying dough.
In a stunning climax, a confusing cascade of reactions involving the various amino acid and sugars produces fresh bread’s wonderful color, and above all its seductive aroma.
The overwhelming complexity of bread-baking stems on the one hand from the fact that countless compounds end up reacting with one another simultaneously, while at the same time, despite an oven temperature perhaps exceeding 200 °C, only the outer layers of dough become this hot. In the interior of the bread the temperature rises much more slowly, reaching at most 100 °C. In other words, the chemical processes at the center differ from those in the outer crumb region, which are in turn distinct from those in the actual crust.
In the final part devoted to this theme we will attempt to provide at least an overview of some of the chemical phenomena involved in the actual baking of bread
References
[7] H.-D. Beelitz, W. Gosch, P. Schieberle, Food Chemistry, Springer Verlag, Berlin, Germany, 2004. ISBN: 978-3-540-69934-7
[8] S. Tamstorf et al., in Chemistry and Physics of Baking (Eds.: J. M. V. Blanshard, P. J. Frazier, and T. Galliard), The Royal Society of Chemistry, London, UK, 1986. ISBN: 978-0851869957
Prof. Klaus Roth
Freie Universität Berlin, Germany.
The article has been published in German in:
and was translated by W. E. Russey.
Our Daily Bread — Part 1
Transformation of ripe ears of grain into a fragrant, aromatic bread borders on the miraculous and behind such a miracle lies chemistry
Our Daily Bread — Part 3
Looks at the chemical reactions during the baking of the bread and the skills trained and experienced bakers need.
Other articles by Klaus Roth published by ChemViews magazine:
- In Espresso — A Three-Step Preparation
Klaus Roth proves that no culinary masterpiece can be achieved without a basic knowledge of chemistry
DOI: 10.1002/chemv.201000003 - In Chocolate — The Noblest Polymorphism
Klaus Roth proves only chemistry is able to produce such a celestial pleasure
DOI: 10.1002/chemv.201000021 - In Sparkling Wine, Champagne & Co
Klaus Roth shows that only chemistry can be this tingling
DOI: 10.1002/chemv.201000047 - In Chemistry of a Hangover — Alcohol and its Consequences
Klaus Roth asks how can a tiny molecule like ethanol be at the root of so much human misery?
DOI: 10.1002/chemv.201000074 - In The Chemist’s Fear of the Fugu
Klaus Roth shows that the chemist’s fear of the fugu or pufferfish extends as far as the distinctive and intriguing poison it carries
DOI: 10.1002/chemv.201000104 - In Chemistry of a Christmas Candle
Klaus Roth explains that when we light a candle, the chemistry we are pursuing is not only especially beautiful, but also especially complex
DOI: 10.1002/chemv.201000133 - In Pesto — Mediterranean Biochemistry
Klaus Roth uncovers the nature of this culinary-chemical marvel, and thereby comes to enjoy it all the more
DOI: 10.1002/chemv.201200001 - In Boiled Eggs: Soft and Hard
Klaus Roth examines an egg on its journey from hen to table to ensure the perfect breakfast egg
DOI: 10.1002/chemv.201200018 - Chemical Secrets of the Violin Virtuosi
What made Stradivari’s violins so special? Klaus Roth looks at the important role of chemistry in Stradivari’s workshop and instruments
DOI: 10.1002/chemv.201200076 - Chemical Production in Compliance with Torah and the Koran
The unusual interface between chemistry and religion, drawing upon examples from Islamic and Judaic law
DOI: 10.1002/chemv.201200088 - A Chemical Examination of the Isenheim Altar: Role Played in History by “Horned Rye”
Takes a scientific look at the Isenheim Altar, which depicts the symptoms and treatment of “St. Anthony’s Fire”, the result of poisoning by ergot alkaloids
DOI: 10.1002/chemv.201200134 - Video Interview with Klaus Roth
See all articles published by Klaus Roth in ChemViews Magazine




