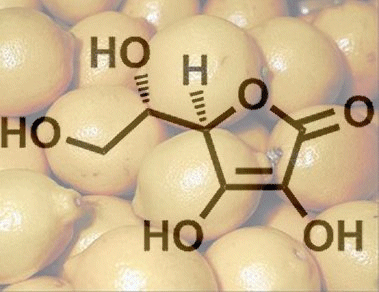
7. Why Can’t Humans Synthesize Ascorbic Acid?
The biosynthesis of ascorbic acid in both plants and animals starts from glucose (Fig. 1), and proceeds through multistep processes to the target L-ascorbic acid. Things went badly for us humans in the course of evolution, as we lack the crucial enzyme, L-gulonolactone oxidase, which is available to animals in general and is required in the final step of the biosynthesis. It was undoubtedly a matter of sheer chance that, for our forefathers roughly 70 million years ago, a mutation occurred in the relevant gene.
Putting it a bit more pointedly, the absence of L-gulonolactone oxidase is for humans the most common genetic disease, as everyone suffers from it. Our ancestors were readily able to handle this genetic deficiency, however, because in their tropical jungle habitat their diets contained ample amounts of vitamin C.
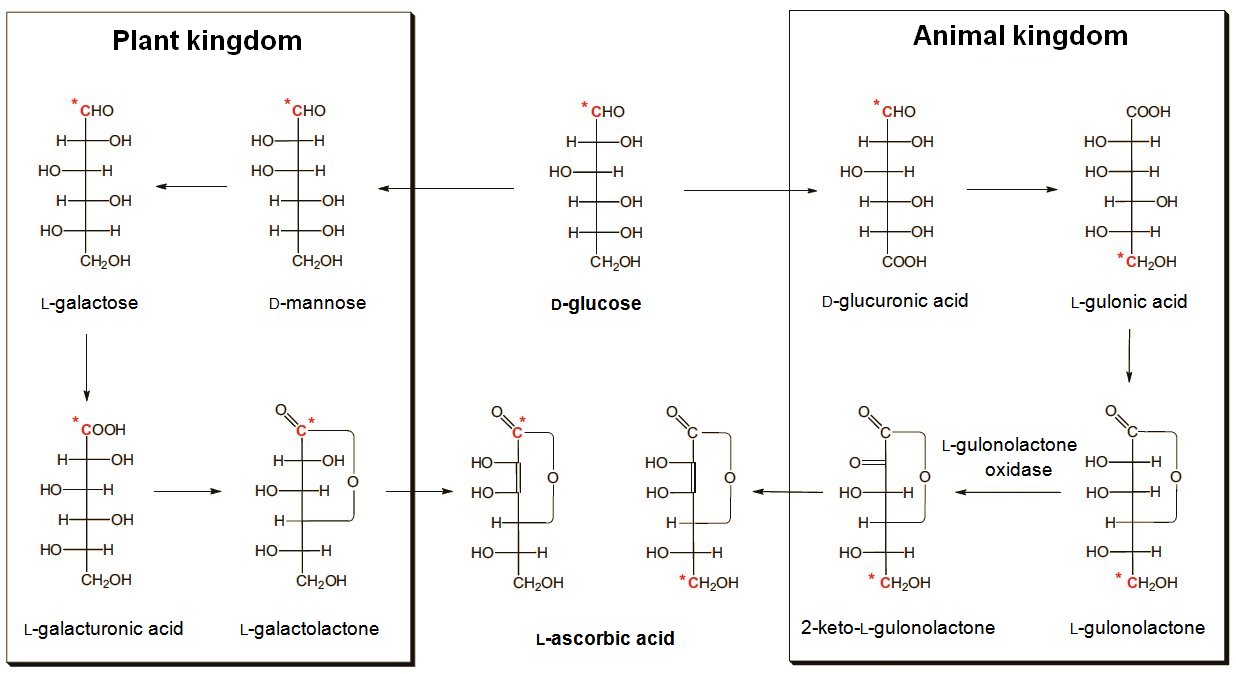
Figure 1. Biosynthesis of ascorbic acid in the animal and plant kingdoms.
It may provide some consolation that people are not alone in having to deal with the absence of L-gulonolactone oxidase; others suffering from the same genetic defect include the rest of the ape family, as well as guinea pigs, fruit bats, the red-vented bulbul (a tropical Asian/African songbird), certain fish, silkworms, and the locust — remarkable company, to say the least! (see Fig. 2)

Figure 2. Some of the other animals incapable of synthesizing vitamin C. (ltr: ape, guinea pig, locust)
Interestingly, as noted above, the synthesis of ascorbic acid begins, for both plants and animals alike with glucose, but from there it proceeds in entirely different ways. Animals carry out the required chain inversion analogously to that in the Reichstein synthesis, whereas plants have elected instead to invert the configurations at two specific stereogenic carbon atoms (C2 and C5) [31, 32], (see Fig. 1).
Actually, the biosynthesis of vitamin C is, from a quantitative standpoint, quite an important process. Every day, a goat, for example, synthesizes an amount of vitamin C that would correspond, for a 70 kg (ca. 150 pound) human, to more than 10 g, or one-third of an ounce. This is for a herbivore that regularly derives a considerable amount of vitamin C from its diet! It was not until recently that it became possible to explain this odd discrepancy.
It turns out that humans are able to take advantage of a shrewdly designed process for recycling ascorbic acid [33]. Thus, the membrane of every human red blood cell contains over 200,000 transport proteins (GLUT1), which normally transport glucose molecules into the cells. With the aid of another protein, stomatin, also in the cell membrane, GLUT1 can be reprogrammed in such a way that, instead of glucose, the red blood cells are loaded with dehydroascorbic acid. This is then reduced to ascorbic acid and, with the help of another transporter, in turn simply released. In this way, dehydroascorbic acid, the oxidation product of ascorbic acid, is very effectively rescued before any irreversible decomposition can occur. Such a transporter trick is not available to the red blood cells of a mouse, for example, as a result of which mice are incapable of recycling dehydroascorbic acid. Any loss of ascorbic acid through the decomposition of dehydroascorbic acid must, thus, be compensated for by the extensive synthesis of ascorbic acid itself.
This suggests that we humans—and presumably the other species without L-gulonolactone oxidase—have effectively compensated for the problem through the extraordinarily sparing utilization of the ascorbic acid we ingest.
8 How Much Vitamin C Does a Person Really Need?
This question has been the subject of longstanding and, to some extent, vehement debate. An adult human maintains a vitamin C reservoir of roughly 2 g. Should this stored quantity fall below 300 mg, typical symptoms of scurvy will soon appear (see Fig. 3).
Thus, in the so-called Iowa Study of 1971, volunteers drawn from a state prison at Fort Madison, Iowa, USA, were first put on a basic diet from which vitamin C was completely absent. This was then supplemented with vitamin C as follows [34]:
|
Days 1–13 |
daily |
75.0 mg of vitamin C |
|
Days 14–110 |
daily |
0.0 mg of vitamin C |
|
Days 110–227 |
daily |
66.5 mg of vitamin C |
The true experiment commenced on day 14, two weeks after establishment of a common daily intake of 75 mg of vitamin C. The subjects were then suddenly shifted to a totally vitamin-C-free regimen. Their bodily vitamin C stores, and thus also the levels of the vitamin in their plasma, fell steadily in the course of the next month. Starting at roughly day 38, the critical plasma level of 0.1 mg/100 ml was crossed, and from day 40, patchy blood spots appeared under the skin of the prisoners. By day 51, this had progressed to swollen, bleeding gums, after day 77, to painful joints, and finally to bleeding in the eyes after day 97. Upon a subsequent daily supply of 66 mg of vitamin C (starting from day 110), their vitamin C stores were gradually refilled, so that plasma levels reached normal values after approximately one month.
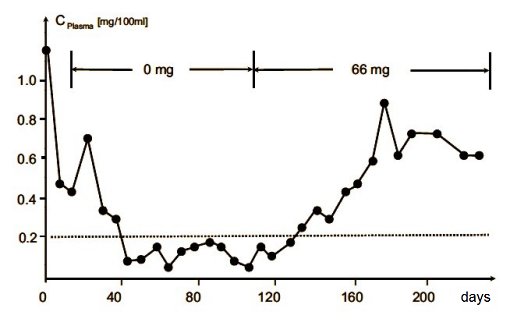
Figure 3. Time course of the levels of vitamin C in the plasma of the participants of the Iowa Study of 1971, in which the volunteers were drawn from a state prison at Fort Madison, Iowa, USA.
Scurvy can be completely prevented with a daily intake of 10 mg of vitamin C (see the information above about goats, a value that will in fact be greatly exceeded in any healthy, balanced, mixed diet) [35]. Isolated cases of scurvy would then be observed only among people who consume virtually no fruit or vegetables, either as a result of poor dietary habits, as with older adults living alone, or in people who have chosen to adhere to extreme, typically fashion-dictated, diets.
The vitamin C requirements of a healthy person are age dependent, and standard recommendations from an array of national health agencies vary only slightly from country to country (Table 1). They all call for increases for certain risk groups:
- Pregnant women and nursing mothers require ca. 110 mg vitamin C/day.
Vitamin C deficiencies are not experienced by breast-fed infants, owing to the high vitamin C content of the breast milk their mothers produce. - The plasma-content of vitamin C decreases with smokers as a function of the number of cigarettes smoked per day [37]. This is because of a corresponding increase in the free-radical concentration in the body and the resulting oxidative load, which in turn leads to a higher vitamin C demand. At more than 20 cigarettes per day, the vitamin C requirement doubles! German, Austrian, and Swiss health authorities recommend for smokers a daily intake of 150 mg.
Table 1. Daily vitamin C requirements.
These values reflect recommended daily dietary levels according to the German, Austrian, and Swiss Society for Nutrition (Deutsche, Österreichische und Schweizerische Gesellschaft für Ernährung) [36]..gif)
9. Linus Pauling and his Megadoses
It is undisputed that, with a daily intake of a mere 10 mg of ascorbic acid, scurvy will not develop. However, Linus Pauling [38–40] posed a rather different question: “How much vitamin C is required daily for maintenance of optimum health, to provide a maximum level of protection against disease, and for optimal support of conventional therapeutic measures?”
The optimal daily vitamin C intake has been a subject of passionate dispute for decades and has been conducted to some extent below-the-belt, scientifically, and with active participation from the tabloid press and so-called medical experts.
To begin with, measuring the optimal supply of a particular compound represents a serious challenge, because the realm between a minimal effective dose and the minimum toxic dose can be very difficult to define experimentally, especially as, for vitamin C in particular, results from animal experiments are only applicable to humans with severe limitations.
Two strategies have been employed in the quest for an optimal dose:
- Experimental studies have shown that, with a daily vitamin C intake of 200 mg, the intracellular level of the compound (i.e., the actual physiologically effective vitamin C concentration) reaches 80 % of its maximum value. Any further intake of vitamin C makes very little difference to the intracellular concentration, with excess vitamin C being almost completely excreted, and thus of no value. On this basis one can conclude that the maximum useful daily dose of vitamin C is in fact 200 mg [41, 42].
- As even in large amounts, vitamin C is not toxic [43, 44], a few investigators have tested the effects of substantially higher daily doses, and argue for alleged enhancements in overall health.
Linus Pauling initiated and popularized the idea that one should in fact consume a gram or more of vitamin C every day. However, whether this really confers health benefits remains to be established clinically. Again, this sounds easier to accomplish than it in fact turns out to be. To make reliable claims it would be necessary for a large number of subjects to participate in a carefully controlled study.
Pauling focused his attention on the common cold. Today, after 60 years of hefty discussion around the question of whether vitamin C has a prophylactic effect with respect to cold-related illness, the record remains a sobering one, as shown by a fairly recent critical meta-analysis [45]:
- Despite prophylactic doses as high as 2 g, the observed frequency of colds remained unchanged. Only in the cases of marathon runners, skiers, and soldiers under considerable low-temperature and physical stress was the frequency of colds reduced, by up to 50 %.
- Prophylactic doses of vitamin C did appear to reduce the length of persistence of such diseases, but only slightly (adults, –8 %, children, –14 %).
Pauling and his students made further claims, attributing additional health-enhancing benefits to vitamin C: detoxification, improved fat metabolism, and, finally, increased lifespan. But no definitive clinical proof could be supplied. Nevertheless, Pauling himself enjoyed the best of health, and continued working almost until the day he died, at the age of 93.
Among all of Pauling’s claims regarding the positive effects of vitamin C, the application of megadoses in cancer therapy was especially controversial. Pauling never asserted that vitamin C alone could cure cancer, instead he recommended daily ingestion of 10 g of vitamin C as a supplement to conventional cancer therapy [38–40]. In a clinical study, Pauling and the Scottish surgeon Ewan Cameron seemingly showed that a daily megadose of 10 g of vitamin C in the final stages of cancer could prolong a patient’s life fourfold. This study became a subject of intense criticism, however, and the results could not be reproduced in a large-scale study at the Mayo Clinic, Rochester, MN, USA. For his part, Pauling doubted the trustworthiness of the Mayo study. The controversy continues to this day, as shown by a relatively recently published article, the associated commentary, and relevant letters to the editor, some of which are quite caustic [46–49].
10 The Role of Vitamin C in Our Nurture
Very few meat and diary products, e.g., liver, lean meat, milk, contain vitamin C, so we must acquire over 90 % of our vitamin C requirement from fruit and vegetables. For Eskimos, these sources are not available in sufficient quantity, however, so they must rely on such unfamiliar delicacies as raw seal liver (18–35 mg/100 g), raw whale skin (35 mg/100 g), and raw cod liver (44 mg/100 g).
But it is not just fruits that contain vitamin C, the same is true of almost all the parts of plants (see Tab. 2).
Table 2. The vitamin C content (mg/100g) of various plants and foods of animal origin..gif)

The data represent benchmarks [19], as the content of vitamin C of these natural products depends on many factors, e.g., variety, geographic origin, climate, soil conditions, time of harvest, storage conditions, etc. Plants recommended as remedies for scurvy over the course of centuries are shown in bold. Some herbs that are familiar as ornamental plants contain an abundant amount of vitamin C, although they play no role in human nutrition. Thus, primroses would offer more vitamin C than blackcurrants [50, 51], even though the latter are often regarded as the ultimate vitamin C source.
Camu-camu (Myrciaria dubia) is a bush found in the Amazon rain forest. It produces a bright-red fruit the size of lemons.
Buttercup should only be consumed prior to blooming, because afterward it produces a toxic component (protoanemonin).
 From a contemporary point of view it must be regarded as a real tragedy that our forebears, out of ignorance, used things like the sandarac tree, buttercup, and scurvy grass in their attempts to cure scurvy. With a single primrose leaf once a day, for example, no seaman would ever have contracted the disease.
From a contemporary point of view it must be regarded as a real tragedy that our forebears, out of ignorance, used things like the sandarac tree, buttercup, and scurvy grass in their attempts to cure scurvy. With a single primrose leaf once a day, for example, no seaman would ever have contracted the disease.
The best way to take advantage of the vitamin C in plants is to eat them fresh and raw. If stored for a long period, finely chopped, or subjected to heat treatment (boiling, frying, steaming) the vitamin C content steadily decreases (Table 3) because ascorbic acid has limited stability in the presence of oxygen. Moreover, it is unfortunately the case that plant material with a high vitamin C content usually also contains a considerable amount of ascorbic acid oxidase, which further accelerates the oxidation.
Table 3. Loss of vitamin C that occurs when various fruits are chopped up [54] and various vegetables are cooked [55, 56].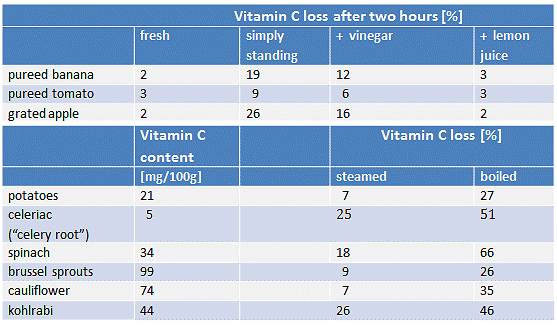
Top of the Table
Chopping up fresh fruit leads at first only to minor losses of vitamin C (2nd column; fresh). After standing for two hours at room temperature, however, the losses become rather large, (3rd column; simply standing). Addition of vinegar to lower the pH can to some extent retard the oxidation processes (4th column; + vinegar). Adding lemon juice reduces the losses further (5th column; + lemon juice), although lemon juice of course adds fresh vitamin C of its own.
Bottom
Due to the thermal stress that accompanies cooking, some vitamin C losses through oxidation processes are inevitable. Steaming and boiling are distinguished primarily through the amount of water involved. Vitamin C is very water soluble (330 g/L), thus the greatest losses in the course of cooking are due to simple dissolution.
For this reason, thermal treatment in particular should be reduced to a minimum. It is important to note, however, that the greatest vitamin C loss related to cooking can probably be attributed to pouring out the water that is used. In addition, a vitamin-C-savvy cook would avoid using copper cookware because copper ions catalyze the oxidation of vitamin C.
Ascorbic acid plays an important role in the industrial processing of foodstuffs, as it is a permissible additive. However, according to European food law its use must be explicitly declared, whether as water-soluble ascorbic acid itself (E 300), sodium ascorbate (E 301), or calcium ascorbate (E 302), but also in such forms as fat-soluble palmitic acid ascorbyl ester (E 304a) and stearic acid ascorbyl ester (E 304b).
These additives are particularly prized; however, not for being significant sources of the vitamin, but instead for improving the shelf life of a product owing to their reducing properties. The broad range of applications of ascorbic acid has been discussed at length elsewhere [52, 53].
Conclusion
It took several centuries before scurvy in its various forms was recognized as a disease caused by a dietary deficiency, and thanks to contributions by many scientists, above all chemists, it has essentially disappeared from the world as we know it. With the help of modern agricultural and dietary science it is now possible to put together a balanced, healthy diet adjusted to suit almost any individual taste.
Perhaps we should be made conscious more often of the fact that the healthiest meals in living memory are what appear on our tables today. And that’s something we can truly celebrate!
References
[31] L. Jaenicke, Chem. Unserer Zeit 1998, 32, 278. DOI: 10.1002/ciuz.19980320507
[32] Glen L. Wheeler, Mark A. Jones, Nicholas Smirnoff, Nature 1998, 393, 365–369. DOI: 10.1038/30728
[33] Amélie Montel-Hagen, Sandrina Kinet, Nicolas Manel, Cédric Mongellaz, Rainer Prohaska, Jean-Luc Battini, Jean Delaunay, Marc Sitbon, Naomi Taylorsend, Cell 2008, 132, 1039–1048. DOI: 10.1016/j.cell.2008.01.042
[34] Robert E. Hodges, James Hood, John E. Canham, Howerde E. Sauberlich, Eugene M. Baker, Am. J. Clin. Nutr. 1971, 24, 432–443. Link
[35] Vitaminversorgung in Deutschland, Stellungnahme der Deutschen Gesellschaft für Ernährung, 2003. Link
[36] Reference values for nutrient intake 2002: Link
[37] C. E. Cross, B. Halliwell, Lancet 1993, 341, 1091–1091. DOI: 10.1016/0140-6736(93)92448-3
[38] L. Pauling, Chem. Brit. 1985, 1, 27
[39] L. Pauling, W. H. Freeman, Vitamin C and the Common Cold, San Francisco, USA, 1970.
[40] L. Pauling, How to live longer and feel better, Avon Books, New York, USA, 1986. ISBN: 9780380702893
[41] Mark Levine, Steven C. Rumsey, Yaohui Wang, Jae Park, Oran Kwon, Nobuyuki Amano, Methods Enzymol. 1997, 281, 425–437. DOI: 10.1016/S0076-6879(97)81048-8
[42] Mark Levine, Steven C. Rumsey, Rushad Daruwala, Jae B. Park, Yaohui Wang, JAMA, J. Am. Med. Assoc. 1999, 281, 1415–1423. DOI: 10.1001/jama.281.15.1415
[43] Seon Hwa Lee, Tomoyuki Oe, Ian A. Blair, Science 2001, 292, 2083–2086. DOI: 10.1126/science.1059501
[44] Vehement reader reactions to the latter: Science 2001, 293, 1993. DOI: 10.1126/science.293.5537.1993c
[45] Robert M Douglas, Harri Hemilä, PLoS Med. 2005, 2(6), e168. DOI: 10.1371/journal.pmed.0020168
[46] Qi Chen, Michael Graham Espey, Andrew Y. Sun, Chaya Pooput, Kenneth L. Kirk, Murali C. Krishna, Deena Beneda Khosh,
Jeanne Drisko, Mark Levine, Proc. Nat. Acad. Sci. USA 2008, 105, 11105–11109. DOI: 10.1073/pnas.0804226105
[47] Balz Frei, Stephen Lawson, Proc. Nat. Acad. Sci. USA 2008, 105, 11037–11038. DOI: 10.1073/pnas.0806433105
[48] Piet Borst, Proc. Nat. Acad. Sci. USA 2008, 105, E95. DOI: 10.1073/pnas.0809328105
[49] Balz Frei, Stephen Lawson, Mark Levine, Qi Chen, Michael Graham Espey, Proc. Nat. Acad. Sci. USA 2008, 105, E96. DOI: 10.1073/pnas.0810008105
[50] Eleri Jones, R. E. Hughes, Phytochemistry (Elsevier) 1983, 22, 2493–2499. DOI: 10.1016/0031-9422(83)80147-2
[51] Eleri Jones, R. E. Hughes, Phytochemistry (Elsevier) 1984, 23, 2366–2367. DOI: 10.1016/S0031-9422(00)80554-3
[52] A. Deifel, Chem. Unserer Zeit 1993, 27, 198–207. DOI: 10.1002/ciuz.19930270405
[53] K. Roth, Unser tägliches Brot. Zum Erntedank, Chem. Unserer Zeit 2007, 41, 400.
and in English: K. Roth, Our Daily Bread, ChemViews Mag. 2013. DOI: 10.1002/chemv.201300016
[54] W. Ternes, Naturwissenschaftliche Grundlagen der Lebensmittelzubereitung, Behr’s Verlag, Hamburg, Germany, 2008. ISBN: 978-3-89947-422-0
[55] J. C. Somogyi, Ernährungsumschau 1975, 22, 42.
[56] A. Meier-Ploeger et al., Ernährungsumschau 1981, 28, 389.
[57] R. Westermeier, Der Gehalt an Vitamin C in verschiedenen Wildkräutern ogv-waldgirmes.de, 2006
[58] S. R. Brown, Scurvy, Thomson Dunne Books, New York, USA, 2004. ISBN: 9780312313920
<Acknowledgments
The authors are grateful to Dr. P. Heinzerling, Hannover, Germany, for supplying certain literature, to Dr. G. Penzlin of Salutas Pharma, Barleben, Germany, for help with tricky matters of nomenclature, and to Dr. P. Winchester for assistance in preparation of the manuscript.
Prof. Klaus Roth
Freie Universität Berlin, Germany.
Dr. Sabine Streller
Freie Universität Berlin, Germany.
The article has been published in German in:
and was translated by W. E. Russey.
It took hundred of years and a long sequence of small advantages and missed opportunities to discover the importance of vitamin C.
When scurvy was identified as a form of malnutrition, an exciting race for the isolation, structure determination, and synthesis of vitamin C started.
Looking at the chemical and physiological properties of vitamin C and at how it intervenes in many biochemical processes
Other articles by Klaus Roth published by ChemistryViews:
- In Espresso — A Three-Step Preparation
Klaus Roth proves that no culinary masterpiece can be achieved without a basic knowledge of chemistry
DOI: 10.1002/chemv.201000003 - In Chocolate — The Noblest Polymorphism
Klaus Roth proves only chemistry is able to produce such a celestial pleasure
DOI: 10.1002/chemv.201000021 - In Sparkling Wine, Champagne & Co
Klaus Roth shows that only chemistry can be this tingling
DOI: 10.1002/chemv.201000047 - In Chemistry of a Hangover — Alcohol and its Consequences
Klaus Roth asks how can a tiny molecule like ethanol be at the root of so much human misery?
DOI: 10.1002/chemv.201000074 - In The Chemist’s Fear of the Fugu
Klaus Roth shows that the chemist’s fear of the fugu or pufferfish extends as far as the distinctive and intriguing poison it carries
DOI: 10.1002/chemv.201000104 - In Chemistry of a Christmas Candle
Klaus Roth explains that when we light a candle, the chemistry we are pursuing is not only especially beautiful, but also especially complex
DOI: 10.1002/chemv.201000133 - In Pesto — Mediterranean Biochemistry
Klaus Roth uncovers the nature of this culinary-chemical marvel, and thereby comes to enjoy it all the more
DOI: 10.1002/chemv.201200001 - Video Interview with Klaus Roth
See all articles by Klaus Roth published by ChemistryViews