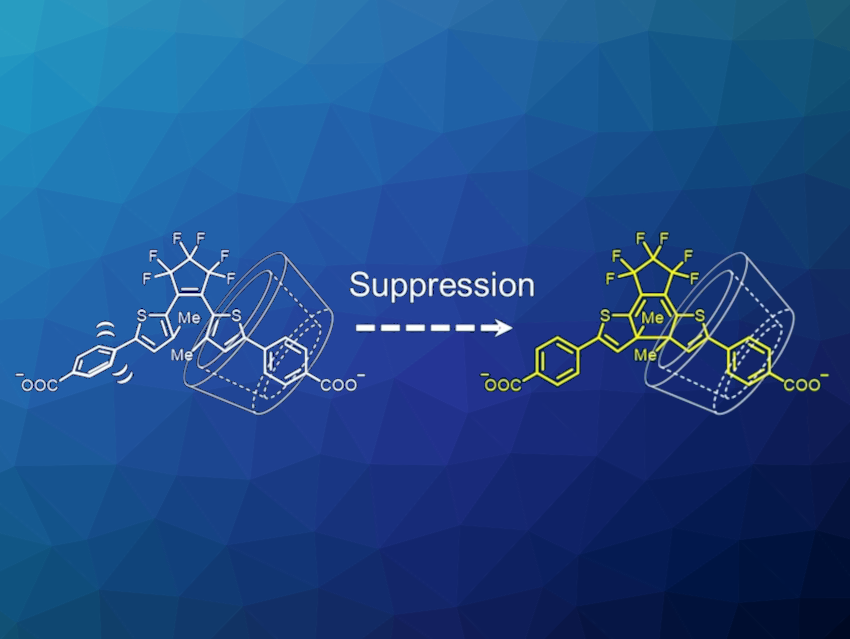
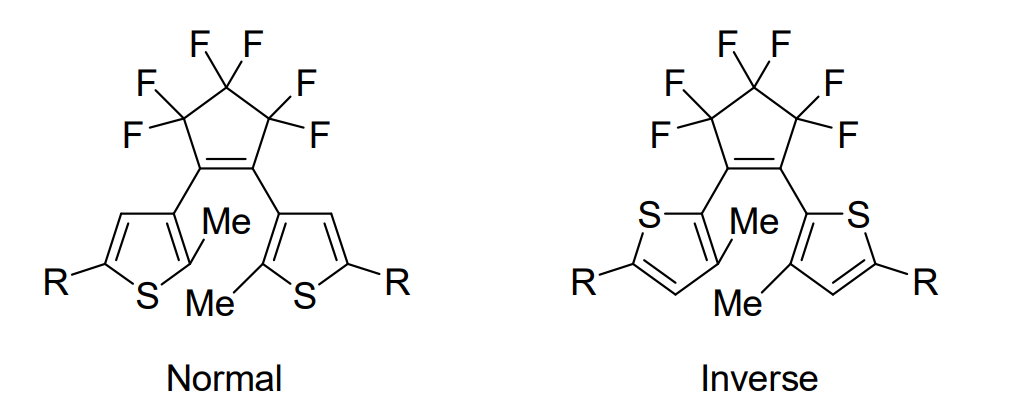
Photochromic molecules can change their color and other physicochemical properties such as fluorescence, electric conductivity, and magnetism in response to light. Diarylethenes with thiophene rings as aryl groups are photochromic compounds, and are classified into “normal type” and “inverse type”, depending on the orientation of thiophene rings (pictured below). These two types show different photochromic reactivity in the solid state. The normal-type molecules usually show both ring-closing and ring-opening reactions even in crystals, while most inverse-type derivatives cannot undergo a photocyclization reaction from the open-ring isomer to the closed-ring isomer in the crystalline state.
Daichi Kitagawa, Seiya Kobatake, Osaka Metropolitan University, Japan, and colleagues have investigated the restriction of molecular conformational change by the host-guest chemistry of cyclodextrins (CDs) to better understand this effect. The team designed and synthesized an inverse-type diarylethene derivative with sodium carbonate groups at the p-position of the lateral phenyl ring and investigated the photochromic behavior in the presence and absence of cyclodextrins with different pore sizes (αCD, βCD, and γCD).
The team found that, interestingly, the photocyclization reaction is suppressed only in the presence of βCD. They propose that the suppression of the photocyclization reactivity is due to the restriction of the rotational motion of the bond between the thiophene and phenyl rings by inclusion into βCD (pictured at the top). In complexes with αCD and γCD, the complexes lead to the encapsulation of other regions of the diarylethenes due to the different pore sizes. Overall, the work could provide useful information for rational design of inverse-type diarylethenes with particular properties.
- Suppression of Photocyclization of An Inverse Type Diarylethene Derivative by Inclusion into β‐Cyclodextrins,
Suganuma Misato, Daichi Kitagawa, Shota Hamatani, Hikaru Sotome, Syoji Ito, Hiroshi Miyasaka, Seiya Kobatake,
ChemPhotoChem 2023.
https://doi.org/10.1002/cptc.202300244