After covering saccharin, the poisonous lead(II) acetate, cyclamate, and aspartame, we look at another artificial sweetener: acesulfame-K.
15. Acesulfame-K
Discovery
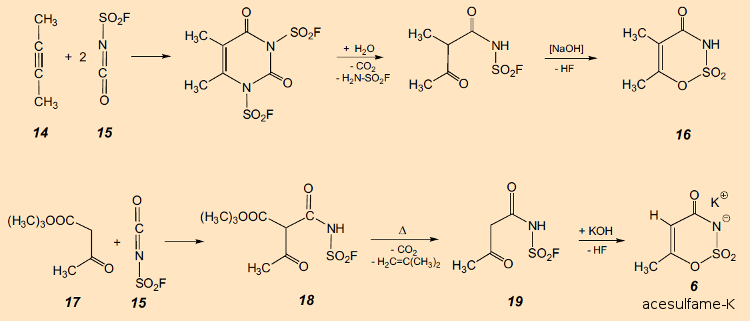
The story of this discovery begins quite fortuitously, as with many other sweetening agents. Karl Clauss, working in 1967 at Hoechst AG in Frankfurt, Germany, carried out a reaction [64] between 2-butyne (14) and fluorosulfonyl isocyanate (15) (see Fig. 21). Incidentally: even the most fearless of chemists would be inclined to show respect for the interaction of two such reactive materials.
|
|
|
Figure 21. Synthesis of a 1,2,3-oxathiazinone (16, top) and synthesis of acesulfame-K (6, bottom). |
Alkaline hydrolysis of the initially formed product led to 16, the first known example of the heterocyclic 1,2,3-oxathiazinone ring system. This result alone would have been of scientific interest, but the true importance of the new compound suggested itself only after Dr. Clauss used a moistened finger in trying to remove a couple of new powdery white spots from his sweater. He was surprised to find that that finger suddenly tasted sweet! Hoechst scientists at once began to explore further this hitherto unknown class of compounds in a search for other especially sweet examples. Karl Clauss and Harald Jensen, in their first relevant publication – which did not appear until several years later – presented not only the accidentally encountered sweet compound 16, but also a host of other promising substances, together with various synthetic approaches to this new class of materials [64].
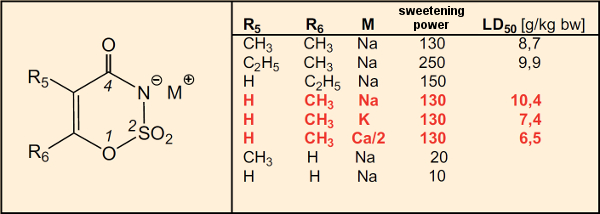
A small selection of compounds drawn from the diligent synthetic efforts of these Hoechst chemists will serve to illustrate the influence of structure on the intensity of sweetness (see Table 2). In the case of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide, acute toxicity studies point out the role played by counter ions (Na, K, Ca; entries in red). The values suggest that, at least in extremely high doses, it is mainly the metal cation that determines overall toxicity. Here, the larger anion plays a much more limited role.
|
Table 2. Influence of structure on intensity of sweetness. |
|
|
Toxicity
Acute toxicity measurements also provided a first indication of the potential commercial value of these sweet-tasting materials. Several of the compounds turned out to have LD50-values > 6g/kg bw (body weight), revealing incredibly high levels of tolerance – in fact significantly above those of common salt (LD50 = 3 g/kg bw) and potassium chloride (LD50 = 2.6 g/kg bw). With regard to the question of which of the many sweet-tasting candidates seemed to have the best chance of commercial success, Clauss and Jensen observed, in 1973 [64]:
|
“Our own focus is especially on the 6-methyl derivative, whose potassium or calcium salts are roughly four times as sweet as cyclamate. In taste tests with a variety of preparations and juices, the purity of their sweet tastes also surpasses that of other derivatives. In addition, their high water solubility offers significant advantages with respect to utilization, since most synthetic sweeteners are in fact only barely satisfactory in this regard. Also, from the standpoint of rate of hydrolysis, these salts meet the demands posed by practical experience. Even in very acidic soft drinks the compounds remain unchanged after months, and with no impairment of the purity of the flavor.” “Acute toxicity values were determined in individual cases, but the compounds subjected to testing turned out to be essentially nontoxic. Nevertheless, these must still be regarded as partial results; we need to await complete toxicological data before passing final judgment over the toxicological suitability of such salts as sweeteners. Moreover, certain aspects of their metabolism remain to be clarified. If the first step involves a ring-opening to give N-acetoacetylamidosulfuric acid, subsequent degradation would lead only to substances already produced naturally in the body. This is another reason why the salts of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide were selected for closer consideration: they are derivatives of the well-known acetoacetic acid.” |
The potassium salt 6 was commonly referred to, starting in 1980, as “acesulfame-K”. This particular compound was singled out due to more than just its sweetening power – other derivatives are significantly sweeter. More important was “the purity of its sweet taste”, which is to say the nature of the overall impression conveyed by its sweetness. One “tastes” the overall quality of a sweetener; sweetness alone is not a sufficient measure, since an unpleasant “overtone”, or a sweetness that remains permanently on the tongue, can be cause for rejection by the consumer.
Acesulfame-K is today manufactured by treatment of the tert-butyl ester of acetoacetic acid (17) with 15 (see Fig. 21). Addition product 18 decomposes at slightly elevated temperatures, under elimination of CO2 and isobutene, into the N-fluorosulfonyl derivative of the amide of acetoacetic acid (19). From this, 6 can, in turn, be obtained via ring closure through treatment with KOH.
Along with a low acute toxicity, numerous studies also support a low chronic toxicity for acesulfame-K. This is due to the fact that acesulfame-K is not actually metabolized in the body, but is instead rapidly, and unchanged, discharged via the kidneys. The only study we reference in detail here is one conducted in the United States in October 2005 in the context of the National Toxicology Program [65]. In this case, mice from two genetically modified strains that develop tumors especially readily were fed a daily dose of acesulfame-K equal to 4–5 g/kg bw for 9 months. This apparently had no effect on their life spans, nor did tumors develop any more frequently than in a control group. In order to properly appreciate the study, it should be noted that the quantity of acesulfame-K delivered orally to the mice was exorbitantly high. It corresponded to daily consumption of 315 g of acesulfame-K for an adult human weighing 70 kg, with the sweetening power of 63 kg of saccharose.
The Joint Expert Committee on Food Additives (JECFA) of the World Health Organization (WHO) established an ADI (Acceptable Daily Intake) value of 15 mg/kg bw/day for acesulfame-K, although in 1991 the SCF (Scientific Committee on Food of the European Commission) instead approved a daily intake of 9 mg/kg bw/d. A request to the SCF that their recommendation be brought in line with the ADI level was denied in 2000, mainly because the retention time of acesulfame-K in dogs had been shown to be much longer than that in rats. The SCF attributed considerable importance to this study with dogs, thus electing to hold to their original 9 mg/kg value [66].
Commercial Use
Acesulfame-K was and remains quite a successful product, in large measure due to its high stability and above all to its synergistic behavior in combination with other sweeteners, especially aspartame. The corresponding calorie-free sugar substitute is marketed under the trade names Sunett and Sweet One. In the European Union, it is recognized under the E-number E950.
References
[64] K. Clauss, H. Jensen, Angew. Chem. 1973, 85, 965–973 (in German). DOI: 10.1002/ange.19730852202
[65] Toxicity studies of acesulfame potassium, U.S. National Institutes of Health, 2005.
[66] E. J. Sinkeldam et al. in Acesulfame-K (Eds: D. G. Mayer, F. H. Kemper), Marcel Dekker Inc., New York, 1991. ISBN: 978-0-8247-8530-7
The article has been published in German as:
- Die Saccharin-Saga,
Klaus Roth, Erich Lück,
Chem. unserer Zeit 2011, 45, 406–423.
DOI: 10.1002/ciuz.201100574
and
- Kalorienfreie Süße aus Labor und Natur,
Klaus Roth, Erich Lück,
Chem. unserer Zeit 2012, 46, 168–192.
DOI: 10.1002/ciuz.201200587
and was translated by W. E. Russey.
The Saccharin Saga – Part 1
The invention of the first artificial sweetener and a lifetime battle for credit
The Saccharin Saga – Part 2
The early industrial production and organized smuggling of saccharin
The Saccharin Saga – Part 3
The health concerns associated with artificial sweeteners
The Saccharin Saga – Part 4
A glance back to ancient Rome, and the most hair-raising of all sweeteners
The Saccharin Saga – Part 5
What’s in your softdrink? – Introducing cyclamate
The Saccharin Saga – Part 6
Aspartame – a sweet dipeptide ester
The Saccharin Saga – Part 7
Acesulfame-K – another successful sweetening agent
The Saccharin Saga – Part 8
Thaumatin – a sweet protein with a licorice aftertaste
The Saccharin Saga – Part 9
Sucrose or Splenda turned into a low-calorie alternative to sucralose or saccharose
The Saccharin Saga – Part 10
Combining sweet cations and anions, and turning bitter compounds into sweeteners
The Saccharin Saga – Part 11
Intelligent synthetic strategies for low-calorie sweeteners
The Saccharin Saga – Part 12
Stevia plant extracts as low-calorie sweeteners
The Saccharin Saga – Part 13
Finding the best mixture of sweeteners to replicate the taste of real sugar
See all articles by Klaus Roth published in ChemistryViews Magazine