After covering saccharin, the poisonous lead(II) acetate, cyclamate, aspartame, acesulfame-K, thaumatin, sucralose, dihydrochalcones, and neotame, we have a look at stevia plant extracts as low-calorie sweeteners.
21. Steviol Glycosides
The genus Stevia (from the Asteraceae family), with its 150–300 species, was originally native to broad subtropical and tropical regions of the Americas. One particular species (“sweetleaf”) from the border area between Paraguay and Brazil has leaves that taste extremely sweet, and with which the aboriginal natives (Guarani peoples) have for centuries sweetened their mate tea (see Fig. 31).
|
|
|
Figure 31. The stevia plant and blossoms illustrated above originated in Paraguay, and represent a rather tall perennial whose leaves are approximately 2–3 cm long. |
This Stevia species was classified by the Swiss botanist M. S. Bertoni in 1899. In 1900, the Portuguese chemist Ovidio Rebaudi isolated its sweet substance for the first time, as a result of which the plant acquired the scientific name Stevia rebaudiana, Bertoni.
21.1. Structure
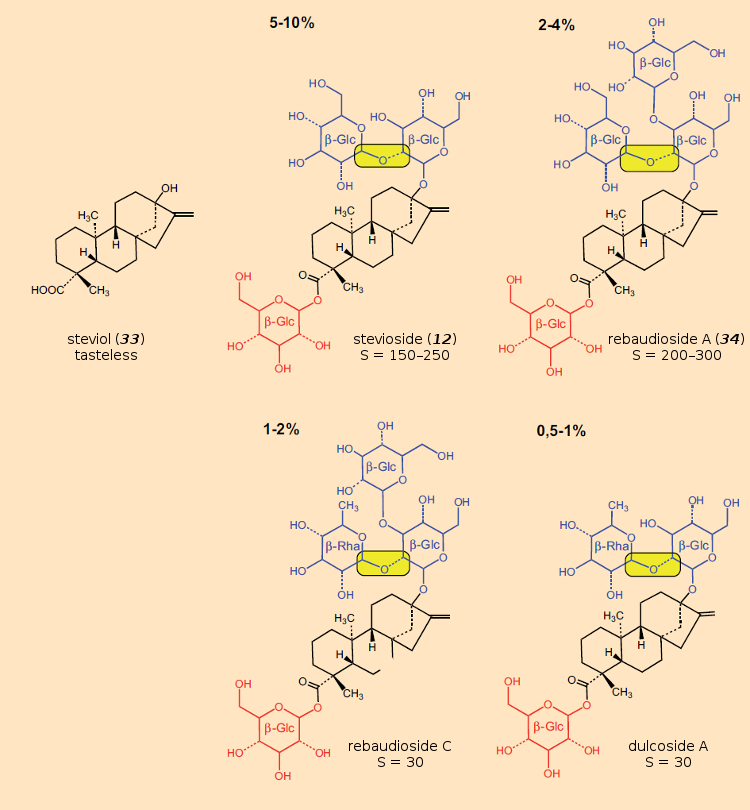
Establishing structures for the components making up this extract occupied chemists over the course of several decades. In 1956, the structure of the primary component, stevioside (12), was first worked out [93]. Dried leaves contain about 8–10 % of sweet-tasting material, over 30 substances have been identified and characterized in the meantime [94]. All of these are derived from one fundamental structure, steviol (33), differing only in the sugar residues incorporated via glycosidic linkages (Fig. 32). From a chemical perspective, the sweet components of Stevia constitute a mixture of steviol glycosides, predominantly stevioside (12) and rebaudioside A (34).
|
|
|
Figure 32. The carboxyl group located on the basic steviol structure is bound to a glucose unit (red). The components differ by the di- or trisaccharide (blue), connected in glycosidic fashion to the hydroxyl group at the other end of the structure. Note that a rare 1,2-glycosic bond (highlighted in yellow) between two of these sugars presented quite a surprise. |
21.2 Toxicology
Although stevia leaves have served for many years as a sweetener – in Japan, for example – gaining approval from the Joint FAO/WHO Expert Committee on Food Additives (JCEFA) and the European Food Safety Authority (EFSA) proved tedious and protracted. Initial animal studies, in fact, pointed to a wide range of harmful characteristics, including teratogenic effects with hamsters, and interference with fertility in male rats. It was further recognized [95] that women of the indigenous Pai-Tavita tribe living in Mato Grosso, one of the Brazilian states, employed the stevia plant as an oral contraceptive. An analogous effect could be confirmed with female rats [96]. Further animal studies showed that stevia leaves and their crude extracts displayed a wide array of physiological effects [97].
Only in retrospect did it become clear that these early studies had all been carried out with impure stevia extracts [98]. In all other more recent studies, on which the sweetener authorization was exclusively based, only highly purified extracts were used, all with a steviol glycoside content of over 95 %. After critical evaluation by many experimentalists, using a host of clinical studies, the JCEFA determined in 2008 that: “steviol glycosides of 95 % purity are harmless for human consumption at levels up to 4 mg/kg bw/d.”
Steviol glycosides were authorized that same year for use as a sweetener in Switzerland, as was rebaudioside A (34) in the United States, followed in France in 2009 by equivalent tentative approval there for two years. Finally, in April 2010, EFSA declared steviol glycosides to be officially harmless from a health standpoint, and recommended their authorization as food additive E 960, with an acceptable daily intake (ADI) value of 4 mg/kg bw/d [99].
This approval for highly purified steviol glycosides was broadened to cover the entire EU in December 2011, but it did not (!) include the stevia plant itself, or its crude extracts. Utilization of the latter for sweetening foods was and is still not permissible in the EU. A petition to include these was reviewed by the Scientific Committee on Food (SCF) as early as 2001, but denied on the grounds that “the available data do not suffice to ensure the harmlessness of such material from a health standpoint.”
21.3. Utilization
Apart from innocuousness, the comprehensive taste sensation is decisive when it comes to a sweetener’s potential commercial success. It became clear that, at higher concentrations, stevioside (12) leaves a bitter metallic, and to some extent licorice-like and long-lasting aftertaste, although this is less pronounced in the case of rebaudioside A (34). Since the authorization for E 960 actually refers to a mixture of steviol glycosides (essentially 12 and 34), S. rebaudiana varieties were soon bred with a higher rebaudioside A (34) content, specifically to improve an extract’s taste profile.
References
[93] E. Vis, H. G. Fletcher, Jr., J. Am. Chem. Soc. 1956, 78, 4709–4710. DOI: 10.1021/ja01599a047
[94] M. Carakostas in Alternative Sweeteners (Ed: L. O’Brien Nabors), CRC Press, Boca Raton, USA, 2012. ISBN: 978-1-4398-4614-8
[95] J. Seidemann, Die Nahrung 1976, 20, 675. DOI: 10.1002/food.19760200614
[96] G. M. Planas, J. Kuć, Science 1968, 162, 1007. DOI: 10.1126/science.162.3857.1007
[97] G. Brahmachari et al., Arch. Pharm. Chem. Life Sci. 2011, 1, 5. DOI: 10.1002/ardp.201000181
[98] A. Abdel-Rahman et al., Toxicol. Sci. 2011, 123, 333. DOI: 10.1093/toxsci/kfr198
[99] European Food Safety Authority, EFSA J. 2010, 8. DOI: 10.2903/j.efsa.2010.1537
The article has been published in German as:
- Die Saccharin-Saga,
Klaus Roth, Erich Lück,
Chem. unserer Zeit 2011, 45, 406–423.
DOI: 10.1002/ciuz.201100574
and
- Kalorienfreie Süße aus Labor und Natur,
Klaus Roth, Erich Lück,
Chem. unserer Zeit 2012, 46, 168–192.
DOI: 10.1002/ciuz.201200587
and was translated by W. E. Russey.
The Saccharin Saga – Part 1
The invention of the first artificial sweetener and a lifetime battle for credit
The Saccharin Saga – Part 2
The early industrial production and organized smuggling of saccharin
The Saccharin Saga – Part 3
The health concerns associated with artificial sweeteners
The Saccharin Saga – Part 4
A glance back to ancient Rome, and the most hair-raising of all sweeteners
The Saccharin Saga – Part 5
What’s in your softdrink? – Introducing cyclamate
The Saccharin Saga – Part 6
Aspartame – a sweet dipeptide ester
The Saccharin Saga – Part 7
Acesulfame-K – another successful sweetening agent
The Saccharin Saga – Part 8
Thaumatin – a sweet protein with a licorice aftertaste
The Saccharin Saga – Part 9
Sucrose or Splenda turned into a low-calorie alternative to sucralose or saccharose
The Saccharin Saga – Part 10
Combining sweet cations and anions, and turning bitter compounds into sweeteners
The Saccharin Saga – Part 11
Intelligent synthetic strategies for low-calorie sweeteners
The Saccharin Saga – Part 12
Stevia plant extracts as low-calorie sweeteners
The Saccharin Saga – Part 13
Finding the best mixture of sweeteners to replicate the taste of real sugar
See all articles by Klaus Roth published in ChemistryViews Magazine

.jpg)